FIXED CTC
ISET® enables label-free enrichment of CTCs and CTMs as intact cells from all types of solid cancers.

The vast majority of white blood cells (WBC) are eliminated by the ISET® filtration system. Larger cells, enriched by ISET® technology, can be analyzed by cytomorphology and by immunostaining characterization.
Each of the 10 spots on the membrane shows the large cells isolated from 1 mL of blood allowing easy counting.
ISET® technique’s high sensitivity is related to its efficiency: 10 mL of blood are diluted with the Rarecells® buffer, incubated for 10 minutes under soft agitation then transferred to the Rarecells® Block and filtered. CTCs and cluster of tumor cells (called circulating tumor microemboli – CTM) enrichment takes 3 minutes or less. CTCs and CTMs are then ready for staining and further analyses. The ISET® sensitivity threshold of one CTC detection in 10mL of blood is unparalleled.
To maintain this high sensitivity, blood has to be treated within 5 hours after collection. This also allows practically no cell loss during filtration.

The vast majority of white blood cells (WBC) are eliminated by the ISET® filtration system. Larger cells, enriched by ISET® technology, can be analyzed by cytomorphology and by immunostaining characterization.
Each of the 10 spots on the membrane shows the large cells isolated from 1 mL of blood allowing easy counting.
ISET® technique’s high sensitivity is related to its efficiency: 10 mL of blood are diluted with the Rarecells® buffer, incubated for 10 minutes under soft agitation then transferred to the Rarecells® Block and filtered. CTCs and cluster of tumor cells (called circulating tumor microemboli – CTM) enrichment takes 3 minutes or less. CTCs and CTMs are then ready for staining and further analyses. The ISET® sensitivity threshold of one CTC detection in 10mL of blood is unparalleled.
To maintain this high sensitivity, blood has to be treated within 5 hours after collection. This also allows practically no cell loss during filtration.
FIXED CTC WORKFLOW
Fixed CTC Applications
After blood filtration, CTCs are enriched on the ten circular filtration areas (spots) of the membrane. Each spot of the membrane can be analyzed independently, therefore the ISET® System can allow multiplexing. The ISET® System is an open system that allows performing several analyses of CTCs (all publications below report on ISET® technology).
Cytopatological staining
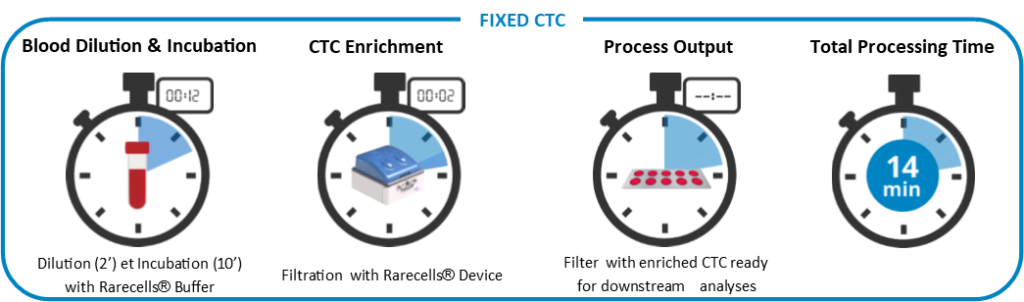
Because the ISET® filtration process isolates intact CTCs, it has been possible to validate those classical cytopathological criteria used in exfoliative cytopathology are reliable for CTC diagnosis (Hofman et al. 2011 Am J Clin Pathol, Hofman et al. 2012 Cytopathology).
Nuclear size equal or larger than 16 microns, irregularity of the nuclear contour, irregularity of the chromatin, presence of a visible cytoplasm, and high nuclear to cytoplasmic ratio (>0.8) are the cytopathological criteria used for CTC diagnosis (Hofman et al. 2011 Clin Cancer Res). ISET® isolates and identifies all types of CTCs and CTMs (Vona et al. 2000 Am J Pathol, Khoja et al. 2014 Melanoma Res, Mazzini et al. 2014 Cancers, Chinen et al. 2014 Oncotargets Ther, Massard et al. 2016 Oncotarget, Long et al. 2016 Cancer Med, Fanelli et al. 2017 Head and Neck).

Because the ISET® filtration process isolates intact CTCs, it has been possible to validate those classical cytopathological criteria used in exfoliative cytopathology are reliable for CTC diagnosis (Hofman et al. 2011 Am J Clin Pathol, Hofman et al. 2012 Cytopathology).
Nuclear size equal or larger than 16 microns, irregularity of the nuclear contour, irregularity of the chromatin, presence of a visible cytoplasm, and high nuclear to cytoplasmic ratio (>0.8) are the cytopathological criteria used for CTC diagnosis (Hofman et al. 2011 Clin Cancer Res). ISET® isolates and identifies all types of CTCs and CTMs (Vona et al. 2000 Am J Pathol, Khoja et al. 2014 Melanoma Res, Mazzini et al. 2014 Cancers, Chinen et al. 2014 Oncotargets Ther, Massard et al. 2016 Oncotarget, Long et al. 2016 Cancer Med, Fanelli et al. 2017 Head and Neck).
IMMUNOcytochemistry (ICC)
Following CTC identification by cytopathology, further CTC characterization is possible through immunocytochemistry (ICC) to identify epithelial-mesenchymal transition (EMT) markers (Hou et al. 2011 Am J Pathol, Lecharpentier et al. 2011 Brit J Cancer), proliferation markers (Hou et al. 2012 J Clin Oncol) and various theranostic markers (Hofman et al. 2013 J Invest Dermatol, Cummings et al. 2014 BMC Cancer).
ISET® enables assessment of PD-L1 expression on CTC, demonstrating concordant PD-L1 staining in tumor tissue and corresponding ISET® membranes from selected NSCLC patients (Ilie et al. 2017 Ann Oncol).

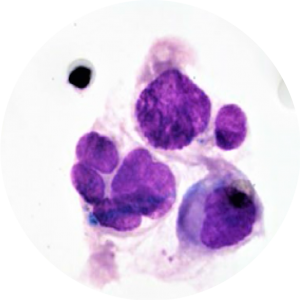
Following CTC identification by cytopathology, further CTC characterization is possible through immunocytochemistry (ICC) to identify epithelial-mesenchymal transition (EMT) markers (Hou et al. 2011 Am J Pathol, Lecharpentier et al. 2011 Brit J Cancer), proliferation markers (Hou et al. 2012 J Clin Oncol) and various theranostic markers (Hofman et al. 2013 J Invest Dermatol, Cummings et al. 2014 BMC Cancer).
ISET® enables assessment of PD-L1 expression on CTC, demonstrating concordant PD-L1 staining in tumor tissue and corresponding ISET® membranes from selected NSCLC patients (Ilie et al. 2017 Ann Oncol).
molecular analyses
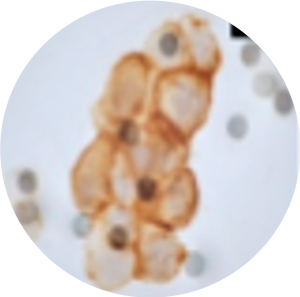
Single-cell targeted Sanger sequencing following laser capture microdissection of individual CTC can help further characterizing them (Vona et al. 2000 Am J Pathol, Vona et al. 2004 Hepatology). Quantitative real-time PCR for assessment of messenger RNA expression (De Giorgi et al. 2010 J Invest Dermatol) and targeted sanger sequencing of KRAS mutations on pools of CTCs (Buim et al. 2015 Cancer Biol Ther) are also possible following ISET®. Single-cell high throughput sequencing following laser capture microdissection and whole genome amplification can help characterizing CTC heterogeneity using either targeted panels or whole exome sequencing (Laget et al. 2017 Plos One).
IMMUNOFLUORESCENCE (IF)
ISET® enables immunofluorescent staining of CTCs, thereby helping automated or semi-automated imaging methods for an efficient and reliable computational analysis of CTCs on ISET® membranes (Pailler et al. 2016 BMC Cancer, Williamson et al. 2016 Nat Commun, Kallergi et al. 2018 Ther Adv Med Oncol, Poruk et al. 2016 Cancer Med).
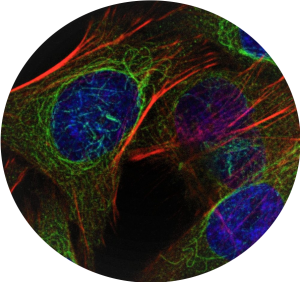

ISET® enables immunofluorescent staining of CTCs, thereby helping automated or semi-automated imaging methods for an efficient and reliable computational analysis of CTCs on ISET® membranes (Pailler et al. 2016 BMC Cancer, Williamson et al. 2016 Nat Commun, Kallergi et al. 2018 Ther Adv Med Oncol, Poruk et al. 2016 Cancer Med).
Fluorescence In situ Hybridization (FISH)
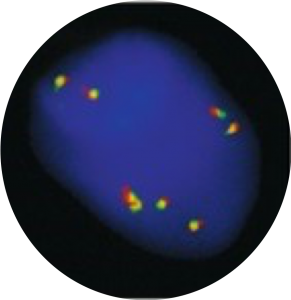
Fluorescence In Situ Hybridization (FISH) applied to CTC recovered by ISET® enables identification of oncogenic fusions, rearrangements and gene amplifications with relevance to therapeutic decision-making and follow-up of potential recurrence (Ilie et al. 2012 Ann Oncol, Pailler et al. 2013 J Clin Oncol, Massard et al. 2016 Oncotarget, Pailler et al. 2017 Cancer Res, Ilie et al. 2018 Clin Chem Lab Med).
ANY QUESTIONS? NEED FOR INFORMATION?
NEED FOR INFORMATION?
NEED FOR INFORMATION?
LIVE CTC
ISET® enables lable-free enrichment of live CTC for further in vitro investigations and analyses.
It is sometimes necessary to isolate live cells without any fixation. This is made possible by our proprietary buffer and live-cell protocol. Live CTC can then be cultured for proliferation and analyses, including RNA analyses or drug sensitivity testing. ISET® is so sensitive that it is capable of recovering one micro-pipetted cell in 10mL of blood.

It is sometimes necessary to isolate live cells without any fixation. This is made possible by our proprietary buffer and live-cell protocol. Live CTC can then be cultured for proliferation and analyses, including RNA analyses or drug sensitivity testing. ISET® is so sensitive that it is capable of recovering one micro-pipetted cell in 10mL of blood.
LIVE CTC WORKFLOW
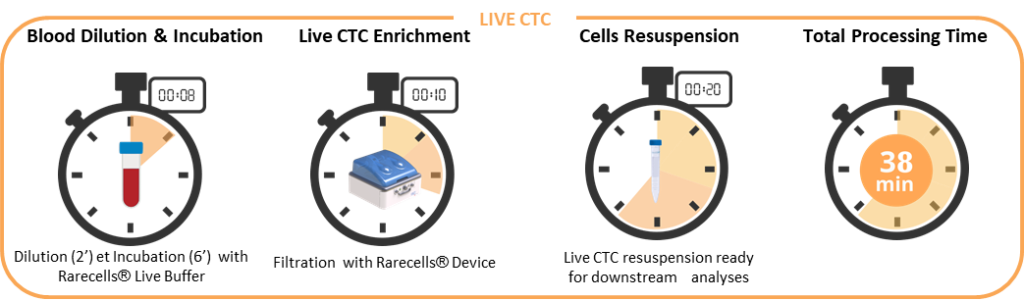
LIVE CTC Applications
After blood filtration, the live CTC are collected in suspension in 15 mL falcon tubes. Each cell resuspension tube can be analysed independently, therefore the Rarecells® System can allow multiplexing for live cells, as listed in the next paragraphs. As live CTC collection is a recently developed application of ISET®, some of the publications cited below do not report on ISET®.
IN VITRO CTC CULTURE
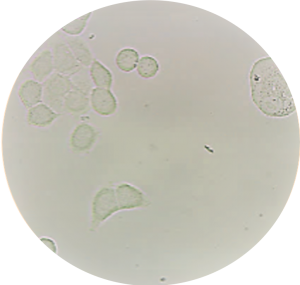
Performances of the ISET® filtration process for live CTC collection were recently assessed thoroughly, demonstrating the unparalleled sensitivity of live-cell collection (Laget et al. 2017 Plos One). Because ISET® allows enrichment of live cells with intact nucleus and cytoskeleton features, in vitro CTC culture is possible (Laget et al. 2017 Plos One).
CTC-DERIVED XENOGRAPHT
Because ISET® allows enrichment of live cells, in vivo studies of CTC through generation of xenographts are possible (Laget et al. 2017 Plos One). CTC-derived xenographts have been proven to enable functional studies such as in vivo drug testing and to allow the generation of CTC-derived cell lines in vitro (Cayrefourcq et al. 2015 Cancer Res).


Because ISET® allows enrichment of live cells, in vivo studies of CTC through generation of xenographts are possible (Laget et al. 2017 Plos One). CTC-derived xenographts have been proven to enable functional studies such as in vivo drug testing and to allow the generation of CTC-derived cell lines in vitro (Cayrefourcq et al. 2015 Cancer Res).
MOLECULAR ANALYSES
Live cell collection by ISET® enables to uncover gene expression profiles of CTC and to study their transcriptomic heterogeneity by quantitative RT-PCR or by high throughput single-cell RNAseq.
IMMUNOLABELLING
Live CTC characterization by immunolabelling following ISET® filtration can guide the identification of specific CTC subsets since live cell collection by ISET® does not impact cell surface markers (Laget et al. 2017 Plos One).
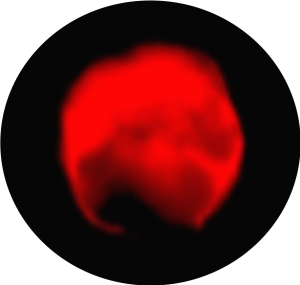

Live CTC characterization by immunolabelling following ISET® filtration can guide the identification of specific CTC subsets since live cell collection by ISET® does not impact cell surface markers (Laget et al. 2017 Plos One).
ANY QUESTIONS? NEED FOR INFORMATION?
NEED FOR INFORMATION?
NEED FOR INFORMATION?
PLASMA
Trends are suggesting that CTC analysis and cfDNA analysis are complementary approaches that could provide a comprehensive understanding of patient cancer.
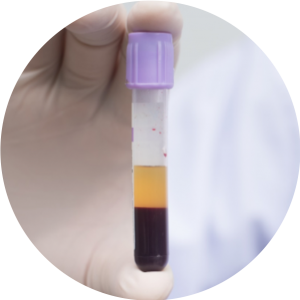
SEPARATED PLASMA ANALYSES
ISET® enables the dual collection of plasma and cellular fraction from a single 10 mL blood sample, without cell loss, to comparatively assess circulating cell-free molecules (DNA, RNA, proteins) and CTC in a given patient (Laget et al. 2017 Plos One).
SEPARATED PLASMA ANALYSES

ISET® enables the dual collection of plasma and cellular fraction from a single 10 mL blood sample, without cell loss, to comparatively assess circulating cell-free molecules (DNA, RNA, proteins) and CTC in a given patient (Laget et al. 2017 Plos One).